Varve Dating and Calibration
The calibration of a glacial varve record, series, or chronology is accomplished by applying numerical or calendar ages to existing varve numbers. The true calendar age of a varve sequence can be obtained in areas where varves can be counted back from the present in modern lakes with varve deposition. A varve sequence might also have events in it of known calendar age, such as beds from flood events or volcanic ash layers that have historic records associated with them. The more usual case is when numerical ages (estimates of true calendar ages) based on radiometric or other techniques are applied to varves. By far the most common numerical ages applied to varve sequences are from radiocarbon (14C) dating.
Although there may be some relatively minor uncertainties associated with 14C ages it is still the most accurate calibration data available in most lacustrine environments where glacial varves occur. With the knowledge of how an organism originally fractionated carbon (based on 13C/12C ratios) and much improved conversions of 14C to calibrated (estimated calendar) years (Stuiver and Reimer, 1993; Stuiver et al., 1998; updated to CALIB 6.0 by Stuiver et al., 2005, see CALIB at http://calib.qub.ac.uk/) it is now possible to obtain highly accurate calibrations of varve sequences.
14C Ages and Varve Calibration
Several circumstances are required for a valid calibration of varve sequences using 14C ages. An important preliminary requirement for an accurate calibration is that the varves being studied should be exactly matched to a varve chronology or they should be measured and have there own numbering system. The exact placement of radiocarbon samples and resulting ages relative to other varves in a varve sequence immediately eliminates one form of uncertainty.
Depositional Lag Times
An additional requirement for accuracy is that the fossil samples do not have a significant lag time associated with their deposition in a lake. Most plant fossils found in glacial lake sediment are from terrestrial sources. As a result there can be a �lag� between the time the organism was alive and when it was finally transported to the lake and deposited with lake sediment. Storage on land, prior to transport and later deposition, may not cause an error in the age of the fossil, but there will be a potential mismatch or lag between the age of the fossil and the younger varve in which the fossil was eventually deposited. Lag times in glacial or periglacial environments may be significant because of the ability of cold environments to protect plant fossils on land from decay for long periods of time.
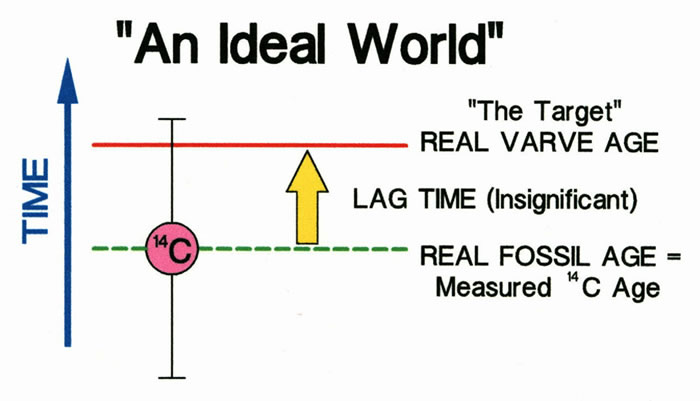
In an ideal world the lag time will be insignificant. Here, insignificant means that the lag time is less than the measurement precision of the 14C age of the plant fossil being dated.
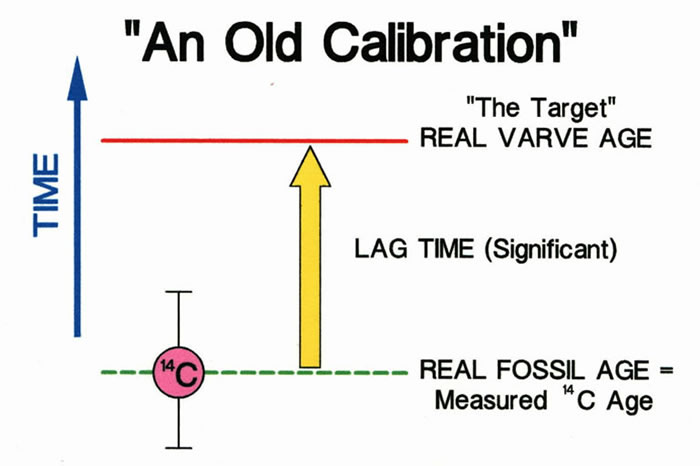
A less than ideal situation is when the lag time is significant (greater than the measurement precision of 14C ages). If this is the case, the lag time will result in a 14C age that overestimates the age of the varve, producing a calibration that is too old.
Eliminating Lag Times
Unfortunately, there is no way to directly determine the span of a lag time for a single fossil. However, there are some general guidelines in choosing material for dating that may help remove or diminish some uncertainties regarding the lag time problem.
- Clumps of organic matter that look like they were eroded from terrestrial settings, such as fragments of peat or gyttja that were eroded from flood plain settings or breeched wetlands, are likely to have lags associated with them. They represent environments where sediment was accumulated on land for some time before being eroded and transported to a lake. They also have the disadvantage of potentially containing the remains of aquatic vegetation and algae, which will produce errors as discussed below. It is recommended that such re-deposited materials be avoided when collecting samples for 14C ages.
- Delicate leaves from terrestrial plants are not as likely to withstand long periods of storage in a terrestrial setting followed by transport as are more hardy fossils such as twigs or pieces of wood. If possible, ages from twigs or wood should be compared to the ages of more delicate fossils found in the same varves for any evidence of a significant lag. That being said, there is always the possibility of leaves having significant lag times or errors of unknown origin.
- Another way to potentially avoid problems associated with a significant lag time is to find fossils in the bottom parts of varve sequences or in varves deposited not too long after deglaciation. It is less likely for a plant fossil with a significant lag time to make its way into lake beds that were deposited a relatively short time after deglaciation because vegetation has not been present on the land surface adjacent to the lake for a very long time. Unless the original advance of ice or a readvance incorporated older plants into glacial deposits that are then eroded after deglaciation, we shouldn�t expect to see plant material predating the time of deglaciation. Unfortunately, it may be difficult, if not impossible, to find fossils in early ice-proximal varves in some glacial varve sequences where the first few centuries of sediment are effectively barren of organic remains.
The "Reservoir Effect"
For radiocarbon dating to provide a calibration, organic material must occur in the varves, preferably in the form of identifiable terrestrial plant remains. 14C ages from terrestrial plants avoid some problems with other materials. Bulk sediment samples and samples of aquatic plants or animals such as the carbonate shells of clams, snails, and ostracodes may yield ages that are too old because of how carbon was fixed by the organism and the potential for the incorporation of “old” carbon from a water body. Many water bodies are not in equilibrium with the atmosphere or have non-atmospheric or recycled sources of carbon in their water columns. In the past, the problems described above have often been lumped together as a “reservoir effect” but their causes are varied and complex.
Shown below is what happens when a fossil that has acquired �old� carbon during its lifetime is used to calibrate a varve chronology. 14C ages that are much older than the true 14C age of a fossil, as a result of the living organism incorporating �old carbon�, are not appropriate for varve calibration. These ages will result in a calibration that is too old and often well outside the uncertainties associated with the precision of 14C measurement. In some cases, errors may become obvious as they are sometimes on the order of 10 kyr or more (Miller and Thompson, 1979). The most reliable ages are from fossils that got their carbon directly from the atmosphere. If you want accurate results, the bottom line is that the 14C concentration of a sample should only be different from the 14C concentration of the atmosphere during the organisms lifetime as a result of fractionation by the organism and subsequent radiometric decay.
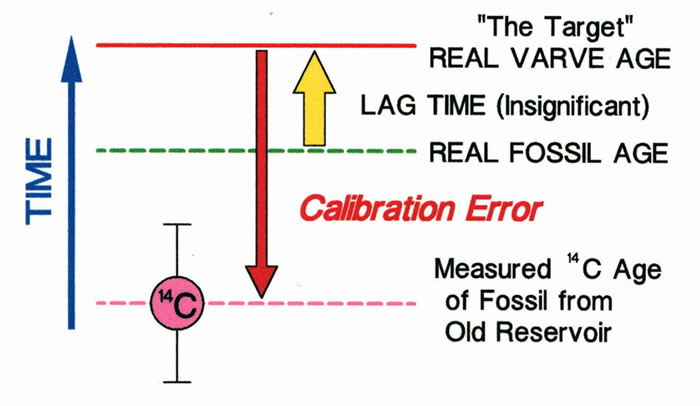
A significant error in radiocarbon ages results with the dating of a fossil of an organism that took up carbon from non-atmospheric sources.
Sample Contamination
One final caution on 14C ages that has sometimes been ignored is the problem of sample contamination. This problem has often been ignored because it is difficult to evaluate until many 14C ages are obtained and the �bad apples� reveal themselves. One must also remember that all plant fossils are in some state of decay, which can introduce contaminants. Most often contamination is the result of younger material added to a sample in a particular field setting. Laboratory procedures may not be fully able to remove the contaminants. Perhaps the most vexing problem in some outcrop studies are pervasive tiny rootlets from modern plants. A fossil that is significantly contaminated with younger material will produce a calibration that is too young even if a lag time might partly offset the error. In the example below, the calibration is too young because a 14C age reflects not only the age of ancient plant remains but also more recent contaminants.
Contamination can also be from old carbon sources as may occur in areas where coal beds are a part of the local bedrock stratigraphy and old coal fragments find there way into the much younger glacial lake sediment.
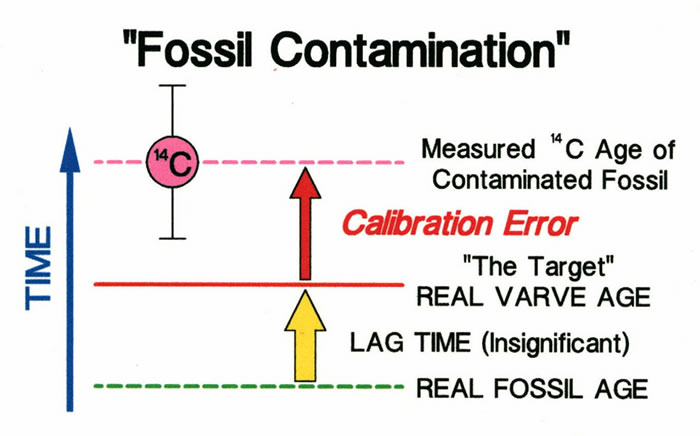
A significant error in radiocarbon ages results when a sample is contaminated by younger material.
References
- Miller, N.G., and Thompson, G.G., 1979, Boreal and western North American plants in the late Pleistocene of Vermont: Journal of the Arnold Arboretum, v. 60, p. 167-218.
- Stuiver, M., and Reimer, P.J., 1993. Extended 14C data base and revised CALIB 3.0 14C age calibration program. Radiocarbon, v. 35, p. 215-230.
- Stuiver, M., Reimer, P.J., Bard, E., Beck, J.W., Burr, G.S., Hughen, K.A., Kromer, B., McCormac, F.G., van der Plicht, J., Spark, M., 1998. INTCAL98 radiocarbon age calibration 24,000 � 0 cal BP. Radiocarbon, v. 40, p. 1041-1083.
- Stuiver, M., Reimer, P. J., and Reimer, R. W. 2005. CALIB 5.0. [program and documentation]. http://calib.qub.ac.uk/calib/


